- 150m về phía Nam, Đường Tây DingWei, Làng Nanlou, Thị trấn Trường An, Khu GaoCheng, Thạch Gia Trang, Hà Bắc, Trung Quốc
- monica@foundryasia.com
Th12 . 27, 2023 13:58 Trở lại danh sách
Metallographic Structure for enamel on cast iron
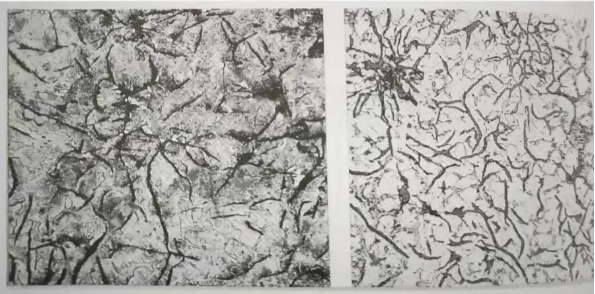
Enamel-coated cast iron cookware is made from a specific composition of cast iron phases, including ferrite and pearlite. Ferrite is a soft and pliable phase, while pearlite combines ferrite and cementite, giving it strength and hardness.
In the process of applying enamel coating to cast iron, it is crucial to understand the metallographic structure to ensure optimal adhesion and durability. This blog post will explore the metallographic structure of cast iron, specifically focusing on the layers that contribute to the successful application of enamel coating.
- 1. Base Layer: Gray Cast Iron
The base layer of cast iron used for enamel coating is typically gray cast iron. This type of cast iron is known for its high carbon content, which gives it excellent strength and wear resistance. It is also characterized by its graphite flakes, which provide good thermal conductivity and reduce brittleness. - 2. Substrate Preparation: Sandblasting and Cleaning
The cast iron surface must be prepared to facilitate proper adhesion of the enamel coating. This often involves sandblasting to remove any impurities or contaminants, followed by thorough cleaning to ensure a clean, smooth surface for the enamel to adhere to. -
For enamel coating, the cast iron should have a balanced ratio of ferrite and pearlite. This composition provides a strong foundation for the enamel to adhere to and ensures the durability of the coating. The ferrite phase helps in absorbing and distributing heat evenly, while the pearlite phase adds strength and resistance to wear.
In addition to ferrite and pearlite, other elements such as carbon, silicon, and manganese play a crucial role. Carbon content should be moderate to provide strength and prevent brittleness. Silicon aids in the adhesion of the enamel coating, while manganese enhances the overall strength and toughness of the cast iron.
-
To summarize, an ideal composition for enamel-coated cast iron cookware includes a balanced ratio of ferrite and pearlite, moderate carbon content, and the presence of silicon and manganese. This composition ensures a durable enamel coating, even heat distribution, and long-lasting performance of the cookware
-
Product introduction of Changan Cast Iron Co., LTD
Tin tứcJan.24,2024
-
The Impact of the Leidenfrost Effect on Non-Stick Properties of Cast Iron Titanium Coated Cookware
Tin tứcJan.24,2024
-
Exploring the Culinary Divide——Cast Iron Casseroles vs Regular Casseroles
Tin tứcJan.03,2024
-
Packaging Workshop Rearranged with Shelving and 3D Storage for Goods
Tin tứcDec.29,2023
-
Cleaning a used cast iron enamel pot can be done effectively with the following steps:
Tin tứcDec.27,2023
-
Metallographic Structure for enamel on cast iron
Tin tứcDec.27,2023